This is an alert
The alert component can be used to highlight certain parts of your page for higher content visibility.Refrigerants & Global Warming
You buy a new HVAC system, or heat pump water heater and there's so much to focus on: price, performance, reliability, availability, installer reputation and financing are probably top of mind, right?
It's understandable if you're less focused on what's internal to the machine itself, like what kind of blower motor it has (brushless? multi-speed?), what piping insulation is used, how wide the ducts are, and which refrigerant is being used.
For a number of reasons, however, as consumers we should be paying a lot more attention to refrigerants, as what refrigerant is used will both impact how equipment ages and – perhaps most importantly – the risk a system poses to the environment and climate change over its lifetime of service. In this post, let's dive into the world of refrigerants, understand what they do, contemplate how we can make the best decisions for the purchases we are making today, and forecast where the industry is going.
Refrigerants and Heat Pumps
What Refrigerants Do
Every heat pump system (HVAC for heating and cooling, a conventional AC only system, your refrigerator, etc) relies on a substance called a “refrigerant” as the main transportation system for the heat it is trying to move (inside to outside, or vice versa). Refrigerants help move heat by undergoing a series of “phase changes” (e.g., liquid to gas, gas to liquid) as it travels through the heat pump system. These phase changes are imposed by changes in pressure; the changes in pressure are imposed by the system's pumps and valves and facilitated by its evaporator coils and heat exchangers.
In order to be effective, refrigerants have a low boiling point. For example, the most commonly used refrigerant used today (called “R-410A”) boils at −48.5 C. As you may recall from high school chemistry (or using your pressure cooker at home), the pressure in the HVAC system helps raise this boiling point closer to ambient temperatures, allowing the pumps and valves of the system to get the substance to “phase change” or go back and forth between liquid (below boiling point) and gas (above) at temperatures that optimize the absorption, transport, and release of heat.
To move the heat, the refrigerant is put in a lower pressure/lower temperature state than its surroundings (this could be just below the inside temperature if you are trying to move heat outside, or - thanks in part to those low boiling point properties discussed before – even below very cold outside temperatures, in the case of trying to move heat indoors. The surrounding air (or water) then tries to find equilibrium, moving the heat into the refrigerant. The now heat-carrying refrigerant is pumped to where it's wanted (or not wanted), phased changed again to be hotter than the surrounding area and “rejected” into the place where that heat is intended.
Properties of a Good Refrigerant
As you can tell, in order to be used effectively and efficiently in a heat pump, refrigerants need a few super powers that a series of compressors and valves can take advantage of: 1) a low boiling point helps refrigerants extract heat from very low temperatures, and use less energy to create the pressure to trigger the phase change; 2) the refrigerant must be fairly stable within the system and potentially thousands upon thousands of phase changes. A refrigerant that corrodes tubing, clogs pumps, or harms valves is not a helpful refrigerant.
Historically, refrigerants have been developed and selected by chemical and heat pump manufacturers based on these “performance” characteristics. The earliest refrigerants were selected only on those characteristics, but were toxic and flammable. Scientists at Frigidaire, GM and Dupont then developed a non-toxic, non-flammable substance called Freon in 1928 that became the preferred refrigerant for decades. But starting in the 70s , environmental impact - specifically impact on the ozone - sparked a realization that refrigerants also needed to take into account their broader impact on the environment, not just their local toxicity. After the initial discovery of ozone depletion occurred in 1974, a series of confirming studies were released in the following decade and in 1987 56 countries agreed to ban the substance all together in what is called the Montreal Protocol. In historical context of other major realizations - like the impact burning carbon-based fuels had on global warming - it was a remarkably swift (13 year) cycle from discovery to complete ban, and today scientists believe the ozone can heal itself within 4 decades (as long as we continue to improve how older systems are maintained/disposed of and China is stopped from illegally manufacturing the substance).
Today's challenge, however, goes beyond the ozone layer, because while today's replacements for Freon do not breakdown O3 in the same way, they are having a devastating impact on global warming at the same time that refrigerants are being used increasingly throughout the industrialized and industrializing world. Couple these trends of industrialization with significantly more political apathy toward global warming relative to what we saw with the ozone crisis, and scientists' best models predict that – if left unaddressed – refrigerants will contribute a whopping 0.5 ℃ to global temperature rise by 2100.
Refrigerant GWP (Global Warming Potential)
Refresher on Global Warming
By now we should all be quite aware of the CO2 story and how it's leading to global warming, but a quick refresher on naturally occurring greenhouse gases and their warming effect may be helpful prior to a more specific discussion of the Global Warming Potential (GWP) of human-made refrigerants. If you're already an expert in global warming, you can skip to the next section.
When contemplating the many miracles of this planet, one of the most significant is our unique atmosphere and the way it maintains a relatively constant temperature near the surface of the planet, including our oceans. One of the most important mechanisms by which Earth achieves this “Goldilocks” temperature is through the fact that certain scarce but powerful molecules in our atmosphere actually vibrate and therefore heat up when our sun's infrared radiation goes through them. In this way, these molecules act like an insulating blanket, trapping and storing the sun's heat in a layer around the Earth's surface, rather than allowing them to radiate back out into the upper atmosphere and back into space. Without this blanket, life on Earth would be like life on Mars, with warm tempuratures during sunlight hours but extreme cold at night.
CO2
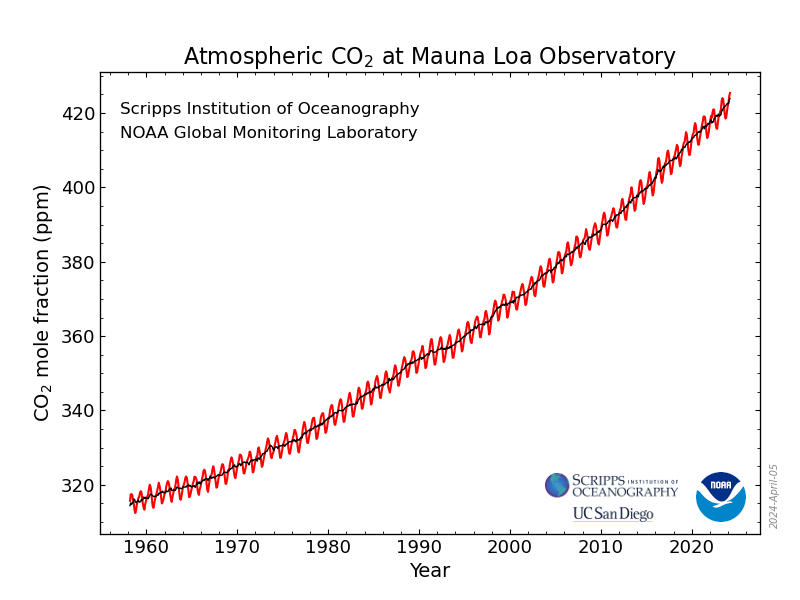
Prior to the industrial revolution, CO2 was mostly in balance in our atmosphere as it was respirated by living organisms (animals, bacteria, fungi) and then consumed again by other living organisms (plants). Major influxes of CO2 could occur after major periods of volcanism or wildfires, but because these were infrequent and over long horizons, excess CO2 in the atmosphere could be absorbed by the oceans, remineralized (permanently turned into other, non-warming molecules) by rocks and soil microbes, or sequestered deep under our oceans as dead biomass. No major change in atmospheric CO2 was ever totally benign and extinction events of course occurred, but these cycles generally occurred on slow enough timescales that Earth and its species could naturally adapt – and naturally evolve around – the changes.
However, starting with the industrial revolution, we began to rapidly overshoot historic levels of CO2 as we started taking carbon from those underground stores and combusting it all while taking CO2 sinks - like forests – and chopping them. Not only did atmospheric CO2 levels start to rise, but they started to rise in mere decades at the rate seen over hundreds of thousands or millions of years historically. While a planet can self-regulate its CO2 over millions of years, humans put Earth and its life in a scientifically impossible position of trying to adapt and evolve in mere decades.
Problems with too much CO2
One key reason the surface (air and oceans) of Earth could support life in the first place is because it became ideally temperate due, in a large part, to a super power of certain molecules in its atmosphere to vibrate (and therefore heat up) when the sun's infrared radiation hits them. If we didn't have sufficient amount of CO2 in our atmosphere, we would look more like Mars, with heat during the day, but extreme cold at night, with no greenhouse gas blanket to keep us just right. But there can be too much of a good thing.
For one, when CO2 concentration of our atmosphere goes up, it doesn't just stay there. Our oceans absorb some of the excess atmospheric gas (in some respects, thankfully), but while this happens they become more acidic and increasingly hostile to critically important life. That life feeds other life, and soon entire food systems start to collapse. Again, as such changes happen in decades timescales (instead of longer timescales) present life doesn't have time to adapt and evolve, and so we simply get a massive and difficult to recover from extinction event rather than a rich evolution of species as we have seen in the past. Species die faster than new ones emerge, disrupting a previous balance and reducing future biodiversity. On land, the rise in temperatures lead to rapid changes in everything from sea level to habitat. Plants/food can no longer grow where it grew for millions of years, but does not have time to spread to new regions (or similar regions do not appear at the rate that old environments are killed). All species, including humans, have to migrate quickly, compete for a different set of resources, creating conflict and ultimately required die-offs. While global temperature rise feels like being boiled alive, the viciousness of weather events can bring the catastrophe into focus overnight, as towns and habitats are wiped off the Earth with high winds, flooding, and wildfire.
Warming Potential of Other Molecules
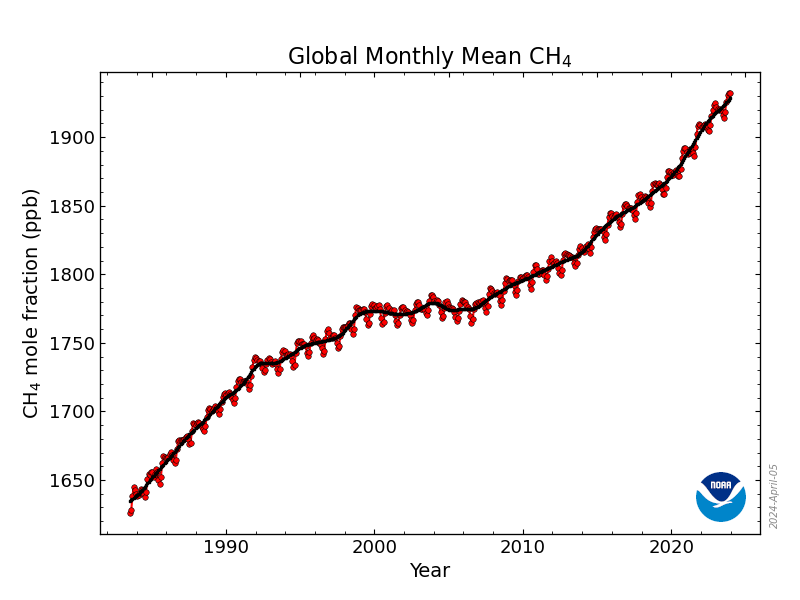
If CO2 was our only concern, we would at least be able to focus on one problem. But alas we have CH4 (aka methane aka “Natural Gas”) and N2 O (aka nitrous-oxide aka “Laughing Gas”) to worry about as well. The good news is that CH4 is 200 times less abundant than CO2 (1930.55 parts per billion (PPB) vs CO2's 425.68 parts per million (PPM)) while N2 O is a mere 337.51 PPB. But the bad news is when solar radiation hits a CH4 molecule it holds onto 100X the amount of energy/heat as the CO2 molecule, making it a peer contributor to global warming. N2 O is even worse, with an ability to trap nearly 300x more than a molecule of CO2 . A molecule's ability to trap this heat is called “ Radiative Efficiency ,” and its ability to trap heat relative to CO2 is called it's “ Global Warming Potential ” (or GWP). GWP is a critical metric we will discuss when we get back to discussing human-made refrigerants. Quickly back to methane and nitrous oxide: while most of our attention in the past several decades has been on CO2 , there is increasing attention being paid to CH4 and N2 O, and for good reason: as we have industrialized methane's extraction and production for energy use, we have been transporting it at very large scales prior to it being burned and turned into CO2 . This supply chain – from the wells themselves all the way through to the storage tanks, piping and appliances in your home – suffers from rampant, hard to detect leakage. And while we can simply reduce how much burning of fossil fuels we do in order to reduce a commensurate amount of CO2 being generated, CH4 now lives in trillions of dollars of infrastructure and will leak as long as it's in there. Meanwhile, N2 O is spewing everywhere we are burning fossil fuels, following a similar story as CO2 , but in addition to that we have built an entire food supply chain – what feeds over 8B people globally – on the manufacture of nitrogen-based fertilizers, as well as other and related land-use and water treatment practices that has put us in a position where 40% of all N2 O emitted each year is coming from human activities.
Lifespan
While Radiative Efficiency / GPW is the primary thing that matters with respect to the greenhouse effect, the second thing to consider with regards to CO2 , CH4 , N2 O or any other molecule in our atmosphere is how long it stays around absorbing the sun's energy before it degrades into a (hopefully) more benign form. The average molecule of CO2 will impact the atmosphere for thousands of years before it becomes remineralized by a rock or otherwise converted into something more harmless. On the flip side, methane starts at 100 times more potent but slowly degrades such that by within 20 years it's “only” ~83 times more potent (from a global warming perspective) than CO2 . In 500 years, methane is “only” 10 times more potent: an irrelevant consolation prize for life on the planet today, but a noteworthy factor as we thing about limiting our harms (and undoing them) for the planet thousands of years from now. Meanwhile, N2 O, like CO2 , barely degrades at all, with a 20 and 100 year GWP of 273 and a 500 year GWP that is still 130 times as warming as CO2 .
As we assess tradeoffs in our human-made emissions, including refrigerants – we must take into account both the Radiative Efficiency of the molecule (or Global Warming Potential relative to CO2 ) and the lifespan of those molecules, and there are no easy ways out.
Warming Potential of Refrigerants
With respect to today's refrigerants, the story isn't any more up-lifting, because despite being produced in much smaller quantities than the naturally occurring + industrially generated molecules we just talked about, research paper after research paper suggests they are on track to contribute an incredible 0.5 ℃ to global temperature rise by 2100. So why can something used in such relative small quantity do so much harm?
In residential HVAC systems, the most commonly used refrigerant since the days of Freon is called R-410A (used in 97% of all 2024 Energy Star systems tracked by Heat Pump Review ), which is a mix of 50% CH2 F2 and 50% CHF2 CF3 ; and boy does the sun's radiation love these molecules. Combined, R-410A has a Global Warming Potential (GWP) of 2,088! That's right: for every molecule manufactured and accidentally released into the atmosphere, the ability of our most popular refrigerant to trap heat is 2,088 times that of CO2 .
Alternatives to R410-A include R-454B, which achieves a meaningful reduction of GWP, down to 466, and R-32 (GWP of 675); however, both are still extremely more dangerous for the environment than the humble and already harmful CO2 .
In other forms of heat pumps there is even more variety of refrigerants and GWPs. Most residental heat pump water heaters and heat pump clothes dryers tracked by Energy Star use R-134A (GWP of 1430), while a handful of water heaters use R-513A (GWP 630) and a handful of clothes dryers use propane (aka R-290, GWP of 3). One novel water heater company, Eco2 Systems, makes a water heater system called SANCO2 which runs on – you guessed it – good ol' CO2 (aka R-744) with a GWP of 1.
Tradeoffs in Cost & Performance
At this point you may be scratching your head: if there are lower GWP refrigerants available – if CO2 itself can be used as a refrigerant – why isn't this a solved problem and why wouldn't everyone just use CO2 (or propane at GWP 3 or R-454B even at GWP 466)?
Everything comes down to the performance characteristics and operating constraints, some of which I outlined before, and all of which impact the overall system cost as well as the more complete emissions picture (a less energy efficient machine may emit more global warming CO2 than the risk of global warming refrigerant leakage in its lifetime).
For instance, Eco2's heat pump water heater can use CO2 as a refrigerant and meet hot water needs of a household – even doing so at competitive “Uniform Energy Factors” (e.g., water heaters' key efficiency metric in the US) with other heat pump water heater systems using conventional refrigerants; but the costs to install are significantly more and the space requirement is also significantly more (tank on the inside, condensing unit on the outside of the home, and specialized piping in between). Propane might be a viable option in a heat pump dryer, but not in an HVAC system that has a pilot light or furnace where flammability could result in tragedy.
So historically engineers (more like the small number of major corporations employing them and controlling the market) have resisted changes in refrigerants until there is regulatory pressure, and instead focused attention on reducing (or masking) leakage, trying to make the environmental dangers of these substances a non-issue, while instead focusing engineering efforts on other parts of their stack.
This dynamic has also been a function of gaps and tensions in our government's system of regulations. On one hand, we want to set really high efficiency standards, like the Dept of Energy's “Energy Star” guidelines, but if the EPA does not simultaneously have the authority to fully regulate refrigerants used by appliance companies to meet those efficiency standards they will naturally lean on the best performing refrigerants not the ones that have the lowest GWP. Thanks to a surprise set of lawmakers, and Biden's EPA, some of these gaps are getting closed.
Regulatory Pressure Toward Lower GWP
In December of 2020, under a Republican Senate and Presidency, the American Innovation and Manufacturing Act (AIM Act) was signed into law.
The AIM Act aligns the US with other global climate leaders (e.g., Europe and other co-signers of the “ Kigali Amendment ” to the original 1987 Montreal Protocol), requiring the industry to move away from high GWP refrigerants in the coming decade. Outwardly, US industry has largely embraced this change , pointing to the past transition from Freon as something that helped stimulate labor and other economic growth in manufacturing, research, and the professional services sectors. Diving a bit deeper into the politics of why this seemingly climate-focused bill sailed through a GOP-controlled Senate and Executive, it's worth noting two totally unrelated facts that the bill's co-sponsors were Senators John Kennedy (R-La.) and Tom Carper (D-Del.) and that the two US-based manufacturers of R-454B (GWP 466) are Dupont spin-off (and Delaware-based) Chemours as well as Honeywell, which has a plant for R-454B in Baton-Rouge . Grease meet wheel.
While the bill outlines a timeline for how quickly manufacturers have to get off R410-A and other high GWP refrigerants (first small milestone is 2025), it remains to be seen how eagerly industry will move to meet these targets. As stated above, 2024's Energy Star heat pumps and air conditioners are still >90% using R410-A despite the legislation being nearly 4 years old and the final EPA rules (which always lag legislation) being nearly 3 years old. In an industry where manufacturers clearly like to “pump” out the same system design again and again, changing refrigerants will likely occur at the very last moment and not before, as each system is tuned to the refrigerant and cannot be 1:1 swapped.
That said, some manufacturers have woken up to the idea that at least some of their customers will care about GWP today. For example, on Earth Day this year Bosch announced two new water-source heat pump models and in their press release the second sentence boasted “The new CL and RL Series heat pumps feature a low global warming potential (GWP) refrigerant [R-454B] in accordance with the forthcoming AIM Act, which will go in effect in January 2025.” Of course a lot of movement may simply be explained by European standards being put into effect faster than US ones, and multi-national companies wanting to monetize their lower GWP portfolio in the US market now (see note on Carrier and CO2 solutions below).
Purchase & Maintenance Decisions
With all of this as background the question remains how we should think about refrigerants in our purchase decisions, what that means in terms of their ongoing maintenance, and what we need to know about their safe disposal.
Considering Refrigerants in Purchase Decision
The clearest implication of this is that when faced with a set of options from your installer, research which refrigerant each will use and – all else being relatively equal – go with the more modern, lower GWP, and CO2 if possible.
Limited CO2 Options
In terms of residential heat pumps, presently Eco2 's hot water heater is the only option available and is a very good option for someone with both the space and the financial flexibility. While generally considered expensive to install, if you need a very high volume of hot water in your household or small business, it would be worth seeing if the system pencils (industry term for makes economic sense) due to the lower operational costs (higher efficiency) and faster backfill of hot water (called First Hour Rating) than other systems. The company's website has a helpful calculator to see how quickly you'll pay yourself back.
Outside of that option, at this point in time there are no residential HVAC systems nor clothes dryers designed around CO2 .
In commercial refrigeration, however, Carrier has invested heavily in CO2 as an option, with their biggest deployments in Europe (over 20,000 units installed), just announcing on May 2nd that they are ready to sell these systems in the US. This is definitely a case of the US benefitting from Europe's more aggressive regulation and the imperitive for multinational companies to simplify and align their business around the lowest GWP options (similarly to how California has impacted the US car market).
We can hope that with the largest residential HVAC manufacturer fully embracing CO2 elsewhere that perhaps they could find a way to use the most greenhouse friendly gas in other systems.
Considering Maintainability at Purchase
There is also a reason to consider purchasing a more expensive model with lower GWP refrigerant beyond altruism. The refrigerant of your system is the one it will need for life. Systems are tuned around a single refrigerant, and so if you would ever need to re-charge your system (of course fixing any leaks first), you would be stuck recharging with the refrigerant you had earlier. A risk is that the refrigerant you select today has higher costs of maintenance going forward because of it being phased out of service (while there is a robust recycling ecosystem for the refrigerants of today, ostensibly a lot of that infrastructure will be transitioned over to R-454B, which seems to be winning this next phase of the HVAC market), and R-32 which has gained popularity in other applications. Certainly safety precautions will remain the highest and therefore most expensive the more harmful the refrigerant.
Maintenance & End of Life (EOL)
Refrigerants are mostly benign for the climate until they leak (caveat is that it does require energy emissions to produce/synthesize them, while CO2 on the other hand is already being captured by power plants and other systems and would benefit from more circular uses).
Leaks
Getting regular maintenance on your HVAC system can help catch leaks early on, but there is a shocking number of people who simply re-charge their systems , either oblivious to or despite the incredible harm they are doing. If you have any system leaking refrigerant (even your car) it is imperative to work with a mechanic who will insist on finding the leak and stopping the leak, vs simply adding more refrigerant in. To do this, they will likely use a “sniffer” (a device with a sensor) while pushing inert nitrogen gas into your system. Leaks can generally be repaired easily, as they are more likely to occur at endpoints where there are a lot of joints, vs in your walls where you usually have long, straight piping. While some mechanics will just add more refrigerant, these are the folks to run far away from, because if they are ignoring regulation or best practice it shows unethical behavior and you don't want them around the rest of your equipment.
Recycling & Destruction
By law, all refrigerants must be reclaimed and disposed of in an approved matter before any refrigerant-consuming appliance is disposed of.
Once recovered from the appliance, presently the most common endpoint for a refrigerant is recycling. To recycle the refrigerant, it's filtered and distilled to get all impurities out (e.g., grease and dust that could have dislodged from the system it previously lived and worked inside), and then put back in a like system.
Refrigerants can also be destroyed using extremely high-heat processes. This endpoint will of course become increasingly common as old systems are retired and there are fewer systems that use high GWP refrigerants to put them back into. Given the energy intensity and general complexity of these processes, the Inflation Reduction Act (IRA) provided $15M in competitive grants to help find new ways to destroy refrigerants.
Conclusion
How we consume energy is driving most climate change, which is why most of our attention is (rightfully) on key heat pump performance metrics in terms of energy consumed. But with the potential to contribute 0.5 ℃ of global warming by 2100, and the potential to cost us significant hidden maintenance costs in the future, the refrigerant deserves our attention. To do this, as informed consumers we should be asking our contractors to optimize for GWP while recommending makes and models. And while our machines are being maintained, we should confirm that our technicians and mechanics are following best practices in terms of fixing leaks, proper reclamation, and proper disposal of all materials. Finally, while we should be glad that the US has adopted a lower GWP regulatory position, we should continue to encourage our elected officials at the local and federal level to make sure the decision is protected no matter who holds power, enforcement is taken seriously, and that research dollars continue to flow into lower GWP options and safer disposal.
As always, I learned a lot researching and writing this article. If you spot any error in my work, or think it would be helpful for me to clarify any of the content, please contact me here .

